Tungsten borides

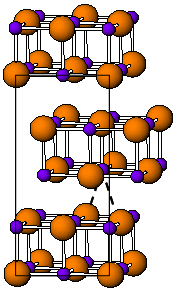
Tungsten borides are compounds of tungsten and boron. Their most remarkable property is high hardness. The Vickers hardness of WB or WB2 crystals is ~20 GPa[1][2] and that of WB4 is ~30 GPa for loads exceeding 3 N.[3]
Synthesis
Single crystals of WB2−x, x = 0.07–0.17 (about 1 cm diameter, 6 cm length) were produced by the floating zone method,[1] and WB4 crystals can be grown by arc-melting a mixture of elemental tungsten and boron.[3]
Structure
WB2 has the same hexagonal structure as most diborides (AlB2, MgB2, etc.).[4] WB has several forms, α (tetragonal), β (orthorhombic) and δ (tetragonal).[2]
Properties
δ-WB and WB2 crystals have metallic resistivities of 0.1 and 0.3 mΩ·cm, respectively. The oxidation of W2B, WB and WB2 is significant at temperatures above 600 °C. The final oxidation products contain WO3 and probably amorphous B2O3 or H3BO3. The melting temperatures of W2B, WB and WB2 are 2670, 2655 and 2365 °C, respectively.[2]
| Material | Vickers hardness (GPa) | Bulk Modulus (GPa) | Melting point (°C) |
|---|---|---|---|
| W2B | 2670 | ||
| WB | ~20 | 2655 | |
| WB2 | ~20 | 2365 | |
| WB4 | ~30 |
References
- ^ a b Otani, S.; Ishizawa, Y. (1995). "Preparation of WB2−x single crystals by the floating zone method". Journal of Crystal Growth. 154 (1–2): 81–84. Bibcode:1995JCrGr.154...81O. doi:10.1016/0022-0248(95)00155-7.
- ^ a b c Okada, S.; Kudou, K.; Lundström, T. (1995). "Preparations and Some Properties of W2B, δ-WB and WB2 Crystals from High-Temperature Metal Solutions". Japanese Journal of Applied Physics. 34 (1): 226–231. Bibcode:1995JaJAP..34..226O. doi:10.1143/JJAP.34.226. S2CID 95651766.
- ^ a b Mohammadi, R.; Lech, A. T.; Xie, M.; Weaver, B. E.; Yeung, M. T.; Tolbert, S. H.; Kaner, R. B. (2011). "Tungsten tetraboride, an inexpensive superhard material". Proceedings of the National Academy of Sciences. 108 (27): 10958–62. Bibcode:2011PNAS..10810958M. doi:10.1073/pnas.1102636108. PMC 3131357. PMID 21690363.
- ^ Woods, H. P.; Wawner, F. E.; Fox, B. G. (1966). "Tungsten Diboride: Preparation and Structure". Science. 151 (3706): 75. Bibcode:1966Sci...151...75W. doi:10.1126/science.151.3706.75. PMID 17842093. S2CID 7686903.
- v
- t
- e
- W(CO)6
- W(PMe3)6
- WSi2
- WCl2
- WI2
- W(OH)2
- W2(hpp)4
- W2O3
- WCl3
- WI3
- W2(OtBu)6
- WC
- WO2
- WS2
- WSe2
- WTe2
- WF4
- WCl4
- WBr4
- WI4
- W2O5
- WBr5
- W2Cl10
- WO2Cl2
- WBr6
- WCl6
- WF6
- WN2
- WO3
- WS3
- WAs2
- WOBr4
- WOCl4
- WOF4
- H2WO4
| Organotungsten(VI) compounds |
|
|---|---|
| Polytungstate salts |
|










